Hemocyanins (Hc), the copper-containing dioxygen carriers, are distributed erratically in two large phyla, Mollusca (for example, octopi and snails) and
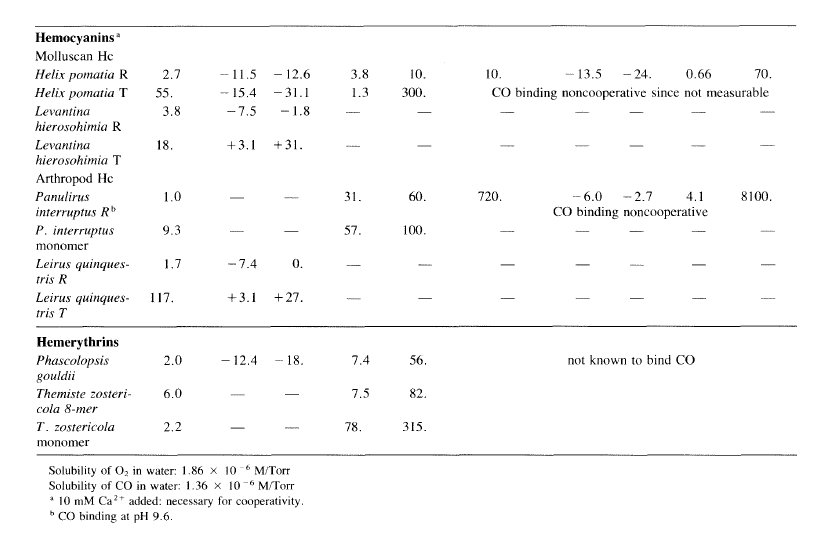
Arthropoda (for example, lobsters and scorpions). The functional form of hemocyanin consists of large assemblies of subunits. 14,15,37 In the mollusc family the subunit has a molecular weight of about 50 kDa and contains two copper atoms. From electron-microscopic observations, hemocyanin molecules are cylindrical assemblies about 190 or 380 A long and 350 Ain diameter comprising 10 or 20 subunits, respectively, for a molecular weight as high as 9 x 10 6 Dalton. In the arthropod family, the subunit has a molecular weight of about 70 kDa with two copper atoms. Molecular aggregates are composed of 6, 12, 24, or 48 subunits. Upon oxygenation the colorless protein becomes blue (hence cyanin from cyanos, Greek for blue). Spectral changes upon oxygenation, oxygen affinities, kinetics of oxygen binding (Table 4.2),4.5,14,15,38 anion binding, and other chemical reactions show that the active site in the phylum Arthropoda and that in Mollusca, although both containing a pair of copper atoms, are not identical. 4, 14
No monomeric hemocyanins, analogous to myoglobin and myohemerythrin (next section), are known. For some hemocyanins the binding of dioxygen is highly cooperative, if calcium or magnesium ions are present, with Hill coefficients as high as n ~ 9. However, the free energy of interaction per subunit can be small in comparison with that for tetrameric hemoglobin; 0.9 to 2.5 kcallmol compared to 3.0 kcallmol. Allosteric effects, at least for a 24-subunit tarantula hemocyanin, can be separated into those within a dodecamer (12 subunits)-the major contributor to overall allostery-and those between dodecamers. 39c This has been termed nested allostery. In contrast to the hemoglobin family, isolated chains have affinities typical of the T-state conformation for hemocyanin. The binding of CO, which binds to only one copper atom, is at best weakly cooperative.
As alluded to above, the distribution of hemocyanins is striking, Among the molluscs exclusive use of hemocyanin as the respiratory protein occurs only with the cephalopods (squid, octopi, and cuttlefish), and in the arthropods only among the decapod (ten-footed) crustaceans (lobsters, shrimp, and crabs). The bivalve molluscs (for example, oysters and scallops) all use small dimeric or octameric hemoglobins. The edible gastropod (snail) Helix pomatia uses hemocyanin, whereas the apparently closely related fresh-water snail Planorbis uses a high-oligomer hemoglobin. Both use a myoglobin as the oxygen-storage protein. The structure of the active site has been extensively probed by EXAFS methods,40,41 and the x-ray crystal structure of a hexameric deoxyhemocyanin is known. 42 Each copper atom is coordinated to three imidazole groups from histidine residues. The pinwheel arrangement of the six subunits, the domain structure of a single subunit, and the domain containing the active site are shown in Figure 4.9.

Tidak ada komentar:
Posting Komentar