Several transition-metal complexes have been observed to catalyze superoxide disproportionation; in fact, aqueous copper ion, Cu2 +, is an excellent SOD catalyst, comparable in activity to CuZnSOD itselfp7 Free aqueous Cu2 + would not itself be suitable for use as an SOD in vivo, however, because it is too toxic (see Section III) and because it binds too strongly to a large variety of cellular components and thus would not be present as the free ion. (Most forms of complexed cupric ion show much less superoxide dismutase activity than the free ion.) Aside from aqueous copper ion, few other complexes are as effective as the SOD enzymes.
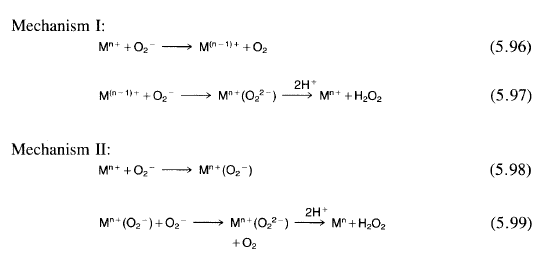
Two mechanisms (Reactions 5.96 to 5.99) have been proposed for catalysis of superoxide disproportionation by metal complexes and metalloenzymes.
In Mechanism I, which is favored for the SOD enzymes and most redox-active metal complexes with SOD activity, superoxide reduces the metal ion in the first step, and then the reduced metal ion is reoxidized by another superoxide, presumably via a metal-peroxo complex intermediate. In Mechanism II, which is proposed for nonredox metal complexes but may be operating in other situations as well, the metal ion is never reduced, but instead forms a superoxo complex, which is reduced to a peroxo complex by a second superoxide ion. In both mechanisms, the peroxo ligands are protonated and dissociate to give hydrogen peroxide.
Analogues for each of the separate steps of Reactions (5.96) to (5.99) have been observed in reactions of superoxide with transition-metal complexes, thereby establishing the feasibility of both mechanisms. For example, superoxide was shown to reduce CuII(phen)z2+ to give Cul(phen)z + (phen = 1,1O-phenanthroline), 106 a reaction analogous to Reaction (5.96). On the other hand, superoxide reacts with CU II(tet b) 2+ to form a superoxo complex 107 (a reaction analogous to Reaction 5.98), presumably because CUII(tet b) 2+ is not easily reduced to the cuprous state, because the ligand cannot adjust to the tetrahedral geometry that Cu1 prefers.
Reaction of superoxide with a reduced metal-ion complex to give oxidation of the complex and release of hydrogen peroxide (analogous to Reaction 5.97) has been observed in the reaction of FeIIEDTA with superoxide. 108 Reduction of a CoIII superoxo complex by free superoxide to give a peroxo complex (analogous to Reaction 5.99) has also been observed.
If a metal complex can be reduced by superoxide and if its reduced form can be oxidized by superoxide, both at rates competitive with superoxide disproportionation, the complex can probably act as an SOD by Mechanism I. Mechanism II has been proposed to account for the apparent catalysis of superoxide disproportionation by Lewis acidic nonredox-active metal ions under certain conditionsY However, this mechanism should probably be considered possible for redox metal ions and the SOD enzymes as well. It is difficult to distinguish the two mechanisms for redox-active metal ions and the SOD enzymes unless the reduced form of the catalyst is observed directly as an intermediate in the reaction. So far it has not been possible to observe this intermediate in the SOD enzymes or the metal complexes.

Tidak ada komentar:
Posting Komentar